
3 One of the consequences will be the gradual decarbonisation of energy sources, which is expected to decrease the overall demand for coal and gas. However, emissions of CO 2 and other greenhouse gases (GHG) into the atmosphere are becoming a global concern and regulations requiring their minimisation are being put in place. For example, conventional methanol production plants can release over 5 t(CO 2)/t(methanol), 2 depending on the feedstock. 1 Typically, syngas is first generated from either natural gas or coal and subsequently converted to methanol, DME or other liquid hydrocarbons via the Fischer–Tropsch process.

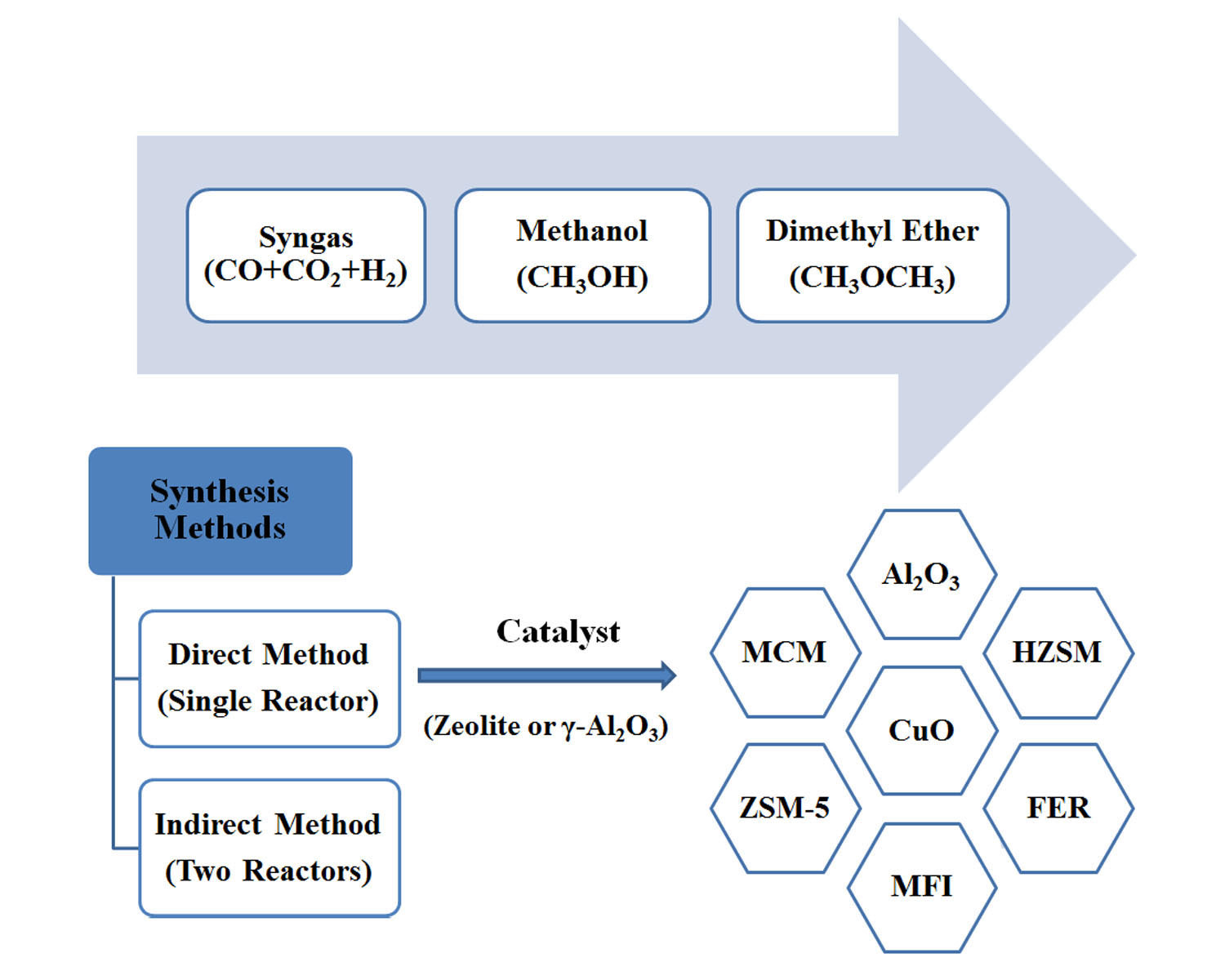
Introduction Presently, dense energy carriers with applications in energy storage, such as methanol and dimethyl ether (DME) are, for economic reasons, derived principally from fossil fuels. CO-based syngas production via high temperature co-electrolysis of H 2O and CO 2, or alternatively high temperature CO 2 electrolysis coupled with the water–gas shift process, was identified as the best technology based on energy consumption and CO 2 utilisation. It was determined that CO 2 could be utilised directly in the direct DME synthesis route, whereas upstream conversion of CO 2 to CO was necessary to achieve effective yields in the methanol/two-step DME systems. Although this system demonstrated the lowest CO 2 emissions per methanol equivalent product with a CO-based feed, the benefits were offset by emissions associated with the upstream conversion of H 2O and CO 2 to H 2 and CO, evaluated in the second part of the study. Based on equilibrium yields at 250 ☌ and 50 bar, the direct DME synthesis system was found to exhibit the highest energy conversion efficiencies with both CO 2- and CO-based syngas. In the first part of the study, the performance of four systems was evaluated and compared in terms of energy efficiency and CO 2 conversion: (1) methanol synthesis system, (2) direct DME synthesis system, (3) two-step DME synthesis system with an interposed syngas separation step between the methanol production reactor and methanol dehydration reactor and (4) two-step DME synthesis system with no separation step between the two reactors. The objective was to establish whether the energy requirements and CO 2 emissions associated with upstream conversion of CO 2 to CO were justified by increased productivity in the methanol/DME systems. The upstream production of a range of syngas feed compositions was simulated using CO 2 and H 2O as the sole chemical building blocks, a requirement motivated by the increasing constraints on permissible CO 2 emissions and the successful adaptation by some industrial methanol plants to the direct utilisation of CO 2. A thermodynamic, model-based, study was carried out to assess the relative performance of methanol and dimethyl ether (DME) synthesis systems using CO- and CO 2-based syngas feeds.


 0 kommentar(er)
0 kommentar(er)
